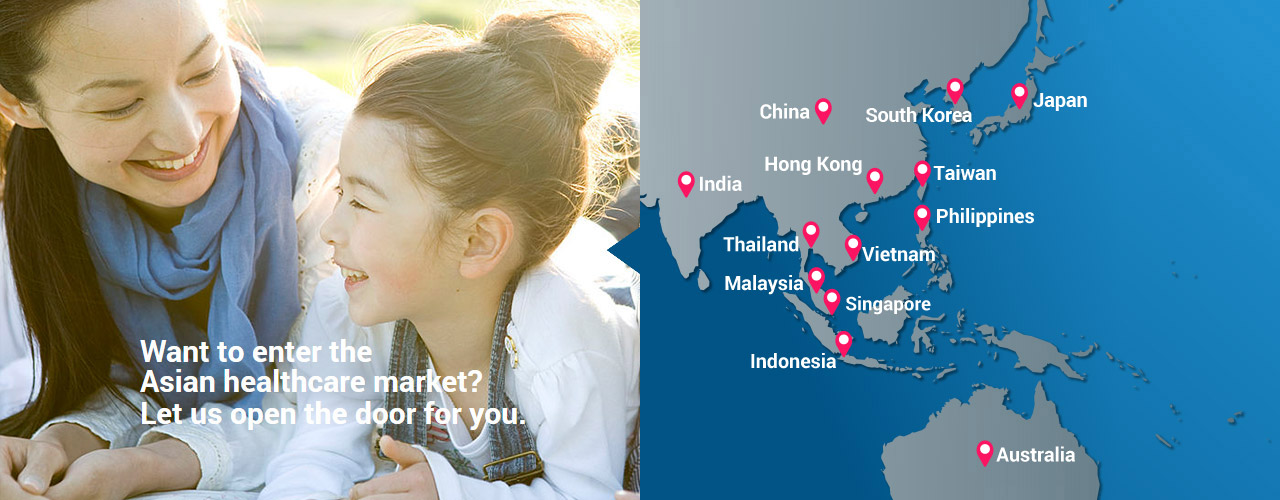
Your Gateway for Clinical Research in Japan, China, and Asia
EPS Corporation an EPS Group Company, serves as a clinical research "Gateway" to Japan, China, and Asia.
As your "Gateway", we provide you with clinical research and market access to Japan, China, and Asia.
More Detail

Share this page