Services
EPS specializes in a wide range of services for nearly any area of therapeutics. We have earned a reputation in the industry as an ideal choice for clinical trials of new drugs, assisting with all aspects of development—from pre-clinical studies to planning and execution of post-marketing initiatives. Our specialists have the experience, knowhow and proven systems that ensure successful implementation of your clinical studies.
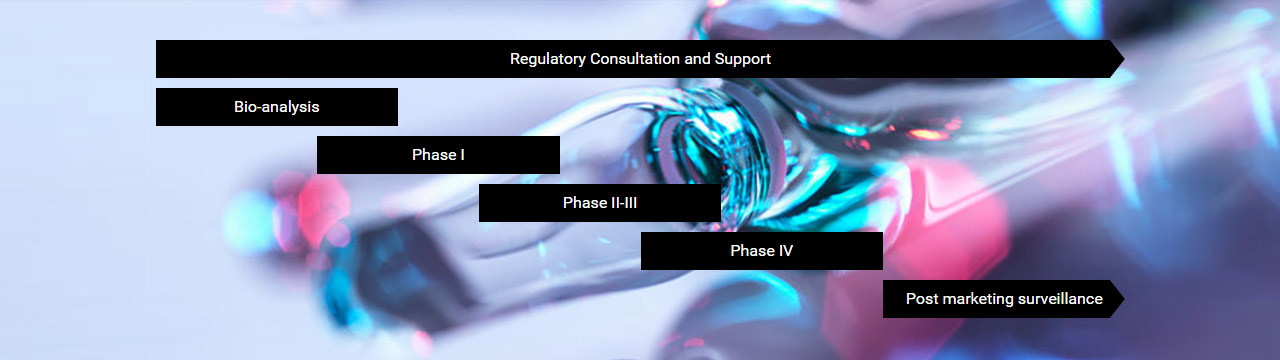
Regulatory Consultation and Support
Post-marketing
surveillance
Our Services
CMC Regulatory Services

In the field of CMC, our expert staff apply highly specialized skills to ...
Contract Sales Organization
The contract sales organization (CSO) provides a series of services and solutions ...
Data Management
Data management consists of three parts: collection, integration, and validation ...
Decentralized Clinical Trials
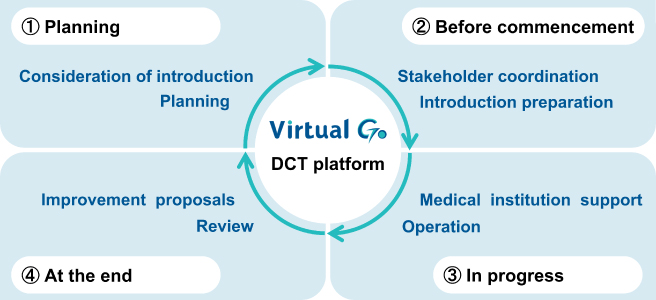
The concept of Patient Centricity in drug development has led to the adoption of Decentralized ...
Insourcing

Because clinical research is conducted in a dynamic environment, it is important ...
Medical Device

Currently, specialized knowledge and procedure are required for Application ...
Medical Writing

From drug to development and clinical trial planning through dossier submission ...
Monitoring

Successful clinical trials rely on efficient clinical monitoring activities. EPS ensures that ...
Patient Recruitment

Successful recruitment has a significant impact on the clinical trial timeline ...
Pharmacovigilance

Pharmacovigilance is commonly known as drug safety. In a contract research ...
Quality Assurance

Quality assurance (QA) plays an important role in the data integrity of clinical research ...
Regulatory Consultation

Delays in product registration can cause a huge loss in market potential ...
Risk-Based Monitoring

Since 2013, the USFDA has recommended risk-based monitoring (RBM) to help ...
Site Management Organization

Proper site management adds positive momentum to successful patient ...
Statistical Analysis

From the development of the statistical analysis plan to the creation of the ...
Therapeutic Areas
Endocrinology & Metabolic
Studies

We will tailor an ideal solution for your specific needs.











Share this page